Different Electrolytes between Li-ion and Li-Po Batteries
Chemical power supply has become an indispensable way of energy storage for people. In the current chemical battery system, lithium batteries are considered to be the most promising energy storage device due to their high energy density, long cycle life, and no memory effect. According to the different electrolyte materials used in lithium-ion batteries, lithium-ion batteries are divided into liquid lithium-ion batteries (LiquifiedLithium-IonBattery, referred to as LIB) and polymer lithium-ion batteries (PolymerLithium-IonBattery, referred to as PLB).

Lithium-ion batteries are generally composed of five parts: cathode material, anode material, diaphragm, electrolyte, and battery shell.
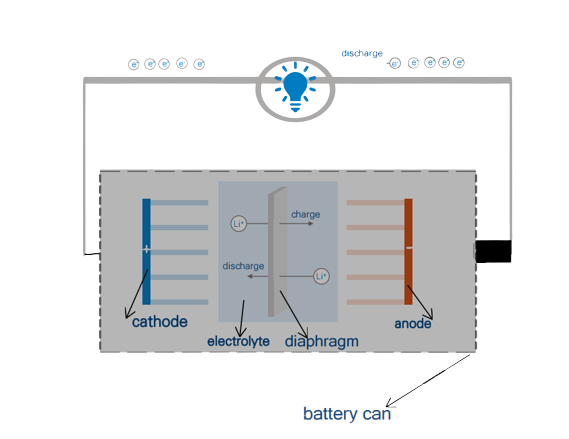
This reversible reaction method commonly known as lithium-ion batteries is a cradle-type reaction. When charging, the lithium ion Li+ runs from the positive electrode through the diaphragm to the negative electrode. Discharge is the opposite. This “swing” forms the basic mechanism of rechargeable lithium-ion batteries.
Discharging Reaction chemical formula
Negative electrode: LixC6-xe–==xLi++6C
Positive electrode: Li1-xCoO2+xLi++xe–==LiCoO2
Total: LixC6+ Li1-xCoO2== LiCoO2+6C

What is a liquid lithium-ion battery?
A lithium-ion battery is a kind of rechargeable battery, which mainly relies on lithium ions to move between the positive and negative electrodes to work. The so-called liquid lithium-ion battery refers to a lithium-ion battery with a liquid electrolyte.

cylindrical li-ion battery

prismatic li-ion battery

li-ion battery
What is a lithium polymer battery?
(Li-polymer, also known as lithium-ion polymer battery), Lipo battery has a variety of obvious advantages such as high energy density, smaller size, ultra-thinness, lightweight, high safety and low cost. It is a new type of battery. In terms of shape, lithium polymer batteries have the characteristics of ultra-thinness, and can be made into batteries of any shape and capacity in accordance with the requirements of various products. The minimum thickness that this type of battery can achieve can reach 0.5mm.

thin

curve

different shapes
Make a Comparison
The Same Points:
1.) The positive and negative electrode materials used in polymer lithium-ion batteries are the same as liquid lithium-ion batteries. The cathode material is divided into lithium cobalt oxide, lithium manganese oxide, ternary material and lithium iron phosphate material, and the negative electrode is graphite.
2.) The working principle of the two is the same: both are charged and discharged through the process of lithium ion embedding and stripping. Among them, the lithium ion is embedded in the negative electrode for charging, and the lithium ion is stripped from the negative electrode for discharging.
Diffrent Point
Liquid lithium-ion batteries use liquid electrolytes.
Polymer lithium-ion batteries are replaced by solid polymer electrolytes, which can be dry or colloidal. Most polymer lithium-ion batteries currently use polymer gel electrolytes.
The most important difference between liquid lithium-ion batteries and polymer lithium-ion batteries is the different electrolytes. This article mainly explains liquid lithium-ion batteries and polymer lithium-ion batteries from the perspective of electrolytes.
What is lithium battery electrolyte?
The lithium battery electrolyte is a chemical that allows an electrical charge to pass between the two terminals. The electrolyte puts the chemicals required for the reaction in contact with the anode and cathode, therefore converting stored energy into usable electrical energy.
Electrolyte is an indispensable and important part of lithium-ion batteries, and it is a necessary condition for lithium-ion batteries to obtain the advantages of high voltage and high cycle performance.
The lithium battery electrolyte is mainly composed of three parts: solvent, lithium salts and addictives.
Function of battery electrolyte
1.) the electrolyte provides part of the active lithium ions, which are used as conductive ions in the charging and discharging process.
2 ) the electrolyte provides an ion channel, or carrier, in which lithium ions can move freely.
This reaction provides power to the connected device, whether it’s a light, a vacuum, or an electric vehicle.
Types of lithium battery electrolyte:
5 kinds of lithium battery electrolyte: Organic Liquid Electrolyte, Solid Polymer Electrolyte, Gel Electrolyte, Room Temperature Ionic Liquid Electrolyte, Inorganic Solid Electrolyte
QUICK Comparison among those five kinds of lithium battery electrolyte
| Organic Liquid Electrolyte | Solid Polymer Electrolyte | Gel Electrolyte | Room Temperature Ionic Liquid Electrolyte | Inorganic Solid Electrolyte | |
|---|---|---|---|---|---|
| States | liquid | Solid | Colloid | liquid | Solid |
| Place of Li+ | unfixed | Relatively fixed | Relatively fixed | unfixed | fixed |
| Concentration of Li+ | low | high | low |
high | Very high |
| Conductivity | high | Pretty low | A bit high | A bit high | A bit low |
| Safety | flammable | excellent | Pretty good | excellent | excellent |
| Price | A bit expensive | A bit expensive | A bit expensive | Very expensive | cheap |
Lithium Battery Design Design Ebook Download(2M, 20 pages, PDF)
Above we have known that the most important difference between liquid lithium-ion batteries and polymer lithium-ion batteries is the different electrolytes. Liquid lithium-ion batteries use liquid electrolytes. While polymer lithium-ion batteries are replaced by solid polymer electrolytes, which can be dry or colloidal.
We are going to illustrate their characteristics clearly.
Organic liquid electrolyte: The electrolyte obtained by dissolving the lithium salt electrolyte in a polar aprotic organic solvent
advantages:
1.)Organic liquid electrolyte has good electrochemical stability,
2.)Organic liquid electrolyte has low freezing point and high boiling point
3.)Organic liquid electrolyte can be used in a wide temperature range.
disadvantages:
1.)Organic solvents of organic liquid electrolyte have a small dielectric constant
2.)Organic solvents of organic liquid electrolyte have a high viscosity
3.)Organic liquid electrolyte has poor ability to dissolve inorganic salt electrolytes
4.)Organic liquid electrolyte has low electrical conductivity
5.)Organic liquid electrolyte are particularly sensitive to trace amounts of water.
6.)Organic liquid lithium batteries are prone to leakage.
7.)The product must use a sturdy metal shell, the shell model and size are fixed which lacks of flexibility.
8.)Poor safety because of the flammablity of organic solvents. Thus the protection measures for the battery must be very perfect.
Gel Electrolyte
The liquid electrolytes have the risk of leakage. And they are prone to thermal runaway and are at risk of combustion or even explosion. After the gel polymer electrolyte diaphragm absorbs the electrolyte, it is fully gelated, which improves the thermal stability and mechanical properties of the electrolyte, not only solves the problem of battery leakage, but also balances the needs of energy storage devices in terms of high ionic conductivity and mechanical properties. Gel polymer electrolyte Lithium-ion batteries add additives such as plasticizers to the solid polymer electrolyte to improve ion conductivity and enable the battery to be used at room temperature.
Gel electrolyte: Liquid plasticizers such as PC, EC, etc. are attracted to the polymer matrix to obtain a solid-liquid composite gel electrolyte.
Advantages of Gel Electrolyte: this ternary electrolyte composed of polymer compounds, lithium salts and polar organic solvents has the properties of both solid electrolytes and liquid electrolytes.
Lithium-ion batteries usually use organic solvents as electrolytes, and such organic solvents are very easy to burn. Once the battery produces high temperature or sparks due to an internal short circuit, the electrolyte will be ignited in an instant and cause the entire battery to explode.
The new idea is to turn the flammable liquid electrolyte into a solid electrolyte to reduce the safety risks caused by the flammable liquid, and at the same time obtain better performance. With the development of new energy vehicles, high-energy density and high-safety batteries have become a must-fight target in the market. Some experts believe that the use of solid electrolytes to replace traditional electrolytes is the only way to essentially improve the safety of lithium batteries.
Solid Polymer Electrolyte
Lithium-ion battery electrolyte is a mixture of polymer and salt. This battery has high ionic conductivity at room temperature and can be used at room temperature.
Compared with liquid electrolytes, the advantages of solid electrolytes
- )Great Safe Performance. Lithium-ion batteries usually use organic solvents as electrolytes, and such organic solvents are very easy to burn. Once the battery produces high temperature or sparks due to an internal short circuit, the electrolyte will be ignited in an instant and cause the entire battery to explode.
- ) High Energy Density.At present, the energy density of lithium batteries used in the market is 200Wh/kg. If a solid electrolyte is used, the energy density of lithium batteries can basically reach 300-400Wh/kg, which has almost doubled.
- )Relatively Light. Compared with liquid batteries, battery packs of the same capacity, solid electrolyte batteries are relatively light. For example, the quality of the ternary lithium battery pack produced by Tesla-Panasonic reaches 900kg, while the quality of the same capacity battery pack produced by Seeo Inc, a solid-state battery startup, is only 323kg, close to one-third of the former.
- ) Strong cycle performance.The solid electrolyte solves the problem of the solid electrolyte interface membrane formed by the liquid electrolyte during the charging and discharging process and the lithium dendrite phenomenon, which greatly improves the cycle and service life of the lithium battery. Under ideal circumstances, the cycle performance is excellent, and it can reach about 45,000 times.
Compared with liquid electrolytes, the disadvantages of solid electrolytes
- ) The interface impedance is too large.Compared with traditional lithium batteries, the solid-solid interface of solid-state electrolyte batteries has weak effective contact between the electrode and the electrolyte, and the transmission dynamics of ions in the solid material are low. In order to avoid the high interfacial impedance caused by the space charge layer, experts continue to conduct experiments, hoping for an early breakthrough.
- ) Fast charging is more difficult. Solid-state electrolyte batteries have LiPON series batteries with very low magnification performance (in fact, the electrolytes of the oxide system generally have poor magnification performance), and solid-state lithium-sulfur batteries with good magnification can also be made based on chalcogenide high-performance electrolytes. But overall, as a power supply, solid-state electrolyte batteries still have many challenges in terms of high magnification performance.
3.) The cost price is high. It is understood that the cost of liquid electrolyte lithium batteries is about 200-300 US dollars/kWh. If the existing technology is used to manufacture a solid electrolyte battery sufficient to power a smart phone, the cost will reach 15,000 US dollars, and the cost of a solid electrolyte battery sufficient to power a car is even more staggering 90 million US dollars.
Conclusion
Since solid electrolytes are used instead of liquid electrolytes, compared with liquid lithium-ion batteries, polymer lithium-ion batteries have the advantages of thinning, any area and any shape, etc., So the battery shell can be manufactured with aluminum-plastic composite film, which can improve the specific capacity of the entire battery; Polymer lithium-ion batteries can also use polymer as the cathode material, and its mass specific energy will be more than 20% higher than the current liquid lithium-ion batteries. Polymer lithium-ion batteries have the characteristics of miniaturization, thinning and lightweight. Therefore, the market share of polymer batteries will gradually increase.
Lithium Battery Design Design Ebook Download(2M, 20 pages, PDF)
============================================================================
Addictive Points
Lithium-ion battery features are as follows:
- ) High energy density: three times that of nickel-cadmium batteries and twice that of nickel-metal hydride batteries
- ) High voltage platform: 3.6v, nickel-metal hydride battery is 1.2V
- ) Low maintenance: no memory effect, no need to discharge regularly
- ) Low self-discharge rate
- ) Environmental protection: no heavy metals

Disadvantages Of Lithium-ion battery:
- ) Requires complex protection circuit
- ) Low discharge magnification: 1C-2C
- ) Will age: the capacity of the stored lithium-ion battery will also decline
- ) Expensive
Commonly used liquid lithium-ion battery structure:
- Cylindrical structure b. Square battery structure c. coin type lithium-ion battery
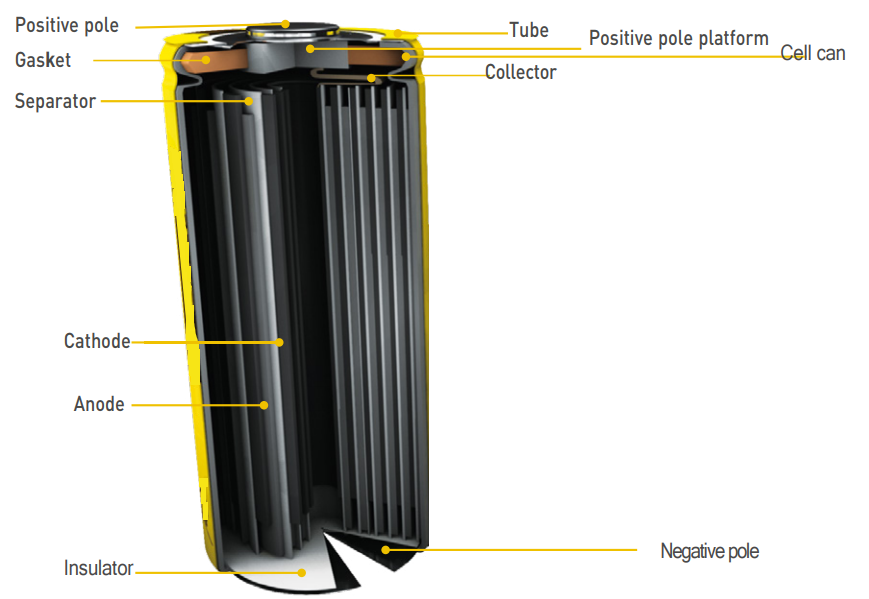
Cylindrical structure

Square battery structure

coin type lithium-ion battery
The characteristics of lithium polymer batteries are as follows:
- ) Safety performance has been improved: a. There is no problem of battery leakage. The battery does not contain liquid electrolyte and uses colloidal solids. b. Resistant to overcharge
- ) Can be made into a thin battery: with a capacity of 3.6V400mah, its thickness can be as thin as 0.5mm. Just like a credit card
- ) The battery can be designed into a variety of shapes.
- ) The battery can be bent and deformed: the polymer battery can be bent up to about 90.
- ) Can be made into a single high voltage: a battery with a liquid electrolyte can only be connected in series with several batteries to obtain a high voltage, while a polymer battery, because it has no liquid, can be made into a multi-layer combination in a single one to achieve a high voltage.
- ) The capacity will be twice that of lithium-ion batteries of the same size.

Prev Article: Lithium Battery: 18650 VS 32650
Next Article: Cylindrical Lithium Battery: 21700 VS 18650



[…] Electrolytes Between Li-Ion and Li-Po Batteries”. DNK. Retrieved from URL https://www.dnkpower.com/different-electrolytes-li-ion-vs-lipo/. (June, […]